- +1-315-215-1633
- sales@thebrainyinsights.com

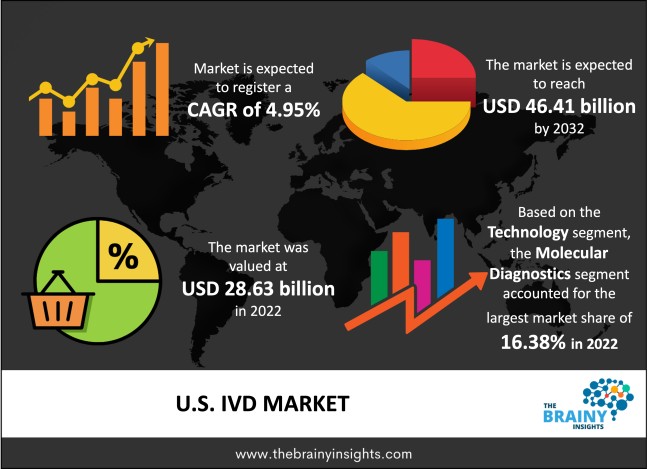
The U.S. IVD market was valued at USD 28.63 Billion in 2022 and grew at a CAGR of 4.95% from 2023 to 2032. The market is expected to reach USD 46.41 Billion by 2032. It is projected that over the forecast period, there will be an exponential decline in the demand for in vitro diagnostics (IVD) to diagnose SARS-CoV-2 infection. However, IVD is a cutting-edge method that offers fresh approaches to detecting and treating diseases. It is projected that IVD testing methods will grow due to the rising incidence of chronic and infectious diseases in the United States.
To offer data for diagnostic, monitoring, and therapeutic reasons, in vitro diagnostics (IVD) is designed for the in vitro analysis of samples produced from the human body. They are employed in diagnosing numerous illnesses, including cancer, cardiovascular, and infectious diseases. The market is primarily driven by the rise in the geriatric population and the incidence of chronic and infectious diseases like diabetes, T.B., and lifestyle diseases. The Affordable Care Act (ACA)'s implementation and a rise in automated lab equipment that enables precise and quick test results have also contributed to the expansion of the IVD market. Additionally, the promotion of point-of-care diagnostics and personalised medicine is projected to promote market expansion. However, the absence of helpful reimbursement policies and strict governmental rules regulating product approval, production, and IVD sales are anticipated to restrain market expansion. The market is anticipated to witness new opportunities due to ongoing R&D efforts connected to IVD. In-vitro diagnostics is a cutting-edge technique that offers new resources for disease detection and therapy. The market for in-vitro diagnostics (IVD) has grown due to the rising incidence of infectious and chronic diseases in the United States. In the U.S., in-vitro diagnostics (IVDs) include much more than simple assays in test tubes and close examination of glass plates. These devices are used to examine patient-provided tissue samples and bodily fluid samples.
Get an overview of this study by requesting a free sample
Worldwide occurrence of chronic diseases and technological advances– In low- and middle-income nations, about 70% of cancer-related deaths occur. The Coronavirus (COVID-19) Pandemic has increased the demand for rapid tests, and the rising incidence of cancer cases and other chronic illnesses is driving demand for self-care tools and Point of Care (POC) diagnostics in the United States for the treatment of persistent illnesses. This is driving demand for oncology-based diagnostics tests and driving the growth of the U.S. IVD Market during the forecast period. Technological advancements are driving the expansion of the U.S. IVD market. A group of methods used for investigating genome and proteome biomarkers are known as molecular diagnostics (Dx). These tests look at specific nucleic acid sequences and how cells express genes as proteins. Rapid molecular tests are innovative diagnostic tests to identify influenza viral RNA or nucleic acids in upper respiratory tract specimens in less than 15 to 30 minutes.
Premarket approval and IVD labelling requirements as specified under the sections of the United States FDA– Premarket Approval (PMA) is a method used by the FDA to evaluate the safety and effectiveness of Class III medical devices through scientific and regulatory evaluation. Since the security of the instrument is rarely linked to contact between the instrument and the patient, IVDs have a unique connection between security and efficiency. For IVD products, the instrument's security is related to the device's function and, more specifically, to the impact of false negative and false positive results on patient health. It is essential to secure Premarket Approval. The 21 CFR 809, Subpart B, In Vitro Diagnostic Products for Human Use, specifies additional labelling standards for in vitro diagnostic products. Manufacturers of IVD products must label their products by labelling requirements before receiving marketing authorisation. These problems are hampering the expansion of the U.S. market for In Vitro Diagnostics.
The technology segment is divided into hematology, molecular diagnostics, microbiology, immunoassays, coagulation, clinical chemistry, and others. The molecular diagnostics segment dominated the market, with a share of around 16.38% in 2022. It is due to the point of care and rapid testing's rising popularity. Growing product launches due to Emergency Use Authorization for several COVID-19 diagnostic procedures have also contributed to the rise.
The application segment is divided into diabetes, cardiology, autoimmune diseases, hematologic diseases, infectious diseases, nephrology, oncology, drug testing, and others. The infectious diseases segment dominated the market, with a share of around 14.77% in 2022. One of the main drivers of this segment's dominance involves the rising COVID-19 testing rate. Additionally, a rise in the prevalence of infectious diseases and the launch of new products have raised segment share in the market.
Leading business organisations are working together to enhance the availability of top-notch, cutting-edge laboratory services for patients and healthcare providers. For instance, in January 2020, Quest Diagnostics and Memorial Hermann Health System teamed up to offer better, more affordable, higher-quality, and more innovative diagnostic services to 21 hospital laboratories in Houston.
| Attribute | Description |
|---|---|
| Market Size | Revenue (USD Billion) |
| Market size value in 2022 | USD 28.63 Billion |
| Market size value in 2032 | USD 46.41 Billion |
| CAGR (2023 to 2032) | 4.95% |
| Historical data | 2019-2021 |
| Base Year | 2022 |
| Forecast | 2023-2032 |
| Segments | Technology, Application |
As per The Brainy Insights, the size of the U.S. IVD market was valued USD 28.63 Billion in 2022 to USD 46.41 Billion by 2032.
U.S. IVD market is growing at a CAGR of 4.95% during the forecast period 2023-2032.
The market's growth will be influenced by worldwide occurrence of chronic diseases and technological advances.
Premarket approval and IVD labelling requirements as specified under the sections of the United States FDA could challenge the market growth.
Key players are F. Hoffmann-La Roche Ltd., Siemens Healthcare GmbH, Qiagen, Quidel Corporation, Abbott, Agilent Technologies, Inc., Becton Dickinson and Company, Bio-Rad Laboratories, Inc., bioMérieux, among others.
1. Introduction
1.1. Objectives of the Study
1.2. Market Definition
1.3. Research Scope
1.4. Currency
1.5. Key Target Audience
2. Research Methodology and Assumptions
3. Executive Summary
4. Premium Insights
4.1. Porter’s Five Forces Analysis
4.2. Value Chain Analysis
4.3. Top Investment Pockets
4.3.1. Market Attractiveness Analysis By Technology
4.3.2. Market Attractiveness Analysis By Application
4.4. Industry Trends
5. Market Dynamics
5.1. Market Evaluation
5.2. Drivers
5.2.1. Worldwide occurrence of chronic diseases and technological advances
5.3. Challenges
5.3.1. Premarket approval and IVD labelling requirements as specified under the sections of the United States FDA
6. U.S. IVD Market Analysis and Forecast, By Technology
6.1. Segment Overview
6.2. Hematology
6.3. Molecular Diagnostics
6.4. Microbiology
6.5. Immunoassays
6.6. Coagulation
6.7. Clinical Chemistry
6.8. Others
7. U.S. IVD Market Analysis and Forecast, By Application
7.1. Segment Overview
7.2. Diabetes
7.3. Cardiology
7.4. Autoimmune Diseases
7.5. Hematologic Diseases
7.6. Infectious Diseases
7.7. Nephrology
7.8. Oncology
7.9. Drug Testing
7.10. Others
8. U.S. IVD Market-Competitive Landscape
8.1. Overview
8.2. Market Share of Key Players in the IVD Market
8.2.1. U.S. Company Market Share
8.3. Competitive Situations and Trends
8.3.1. Product Launches and Developments
8.3.2. Partnerships, Collaborations, and Agreements
8.3.3. Mergers & Acquisitions
8.3.4. Expansions
9. Company Profiles
9.1. F. Hoffmann-La Roche Ltd.
9.1.1. Business Overview
9.1.2. Company Snapshot
9.1.3. Company Market Share Analysis
9.1.4. Company Product Portfolio
9.1.5. Recent Developments
9.1.6. SWOT Analysis
9.2. Siemens Healthcare GmbH
9.2.1. Business Overview
9.2.2. Company Snapshot
9.2.3. Company Market Share Analysis
9.2.4. Company Product Portfolio
9.2.5. Recent Developments
9.2.6. SWOT Analysis
9.3. Qiagen
9.3.1. Business Overview
9.3.2. Company Snapshot
9.3.3. Company Market Share Analysis
9.3.4. Company Product Portfolio
9.3.5. Recent Developments
9.3.6. SWOT Analysis
9.4. Quidel Corporation
9.4.1. Business Overview
9.4.2. Company Snapshot
9.4.3. Company Market Share Analysis
9.4.4. Company Product Portfolio
9.4.5. Recent Developments
9.4.6. SWOT Analysis
9.5. Abbott
9.5.1. Business Overview
9.5.2. Company Snapshot
9.5.3. Company Market Share Analysis
9.5.4. Company Product Portfolio
9.5.5. Recent Developments
9.5.6. SWOT Analysis
9.6. Agilent Technologies, Inc.
9.6.1. Business Overview
9.6.2. Company Snapshot
9.6.3. Company Market Share Analysis
9.6.4. Company Product Portfolio
9.6.5. Recent Developments
9.6.6. SWOT Analysis
9.7. Becton Dickinson and Company
9.7.1. Business Overview
9.7.2. Company Snapshot
9.7.3. Company Market Share Analysis
9.7.4. Company Product Portfolio
9.7.5. Recent Developments
9.7.6. SWOT Analysis
9.8. Bio-Rad Laboratories, Inc.
1.1.1. Business Overview
1.1.2. Company Snapshot
1.1.3. Company Market Share Analysis
1.1.4. Company Product Portfolio
1.1.5. Recent Developments
1.1.6. SWOT Analysis
9.9. bioMérieux
9.9.1. Business Overview
9.9.2. Company Snapshot
9.9.3. Company Market Share Analysis
9.9.4. Company Product Portfolio
9.9.5. Recent Developments
9.9.6. SWOT Analysis
List of Table
1. U.S. IVD Market, By Technology, 2019-2032 (USD Billion)
2. U.S. Hematology, IVD Market, 2019-2032 (USD Billion)
3. U.S. Molecular Diagnostics, IVD Market, 2019-2032 (USD Billion)
4. U.S. Microbiology, 2019-2032 (USD Billion)
5. U.S. Immunoassays, 2019-2032 (USD Billion)
6. U.S. Coagulation, IVD Market, 2019-2032 (USD Billion)
7. U.S. Clinical Chemistry, 2019-2032 (USD Billion)
8. U.S. Others, 2019-2032 (USD Billion)
9. U.S. IVD Market, By Application, 2019-2032 (USD Billion)
10. U.S. Diabetes, IVD Market, 2019-2032 (USD Billion)
11. U.S. Cardiology, IVD Market, 2019-2032 (USD Billion)
12. U.S. Autoimmune Diseases, IVD Market, 2019-2032 (USD Billion)
13. U.S. Hematologic Diseases, IVD Market, 2019-2032 (USD Billion)
14. U.S. Infectious Diseases, IVD Market, 2019-2032 (USD Billion)
15. U.S. Nephrology, IVD Market, 2019-2032 (USD Billion)
16. U.S. Oncology, IVD Market, 2019-2032 (USD Billion)
17. U.S. Drug Testing, IVD Market, 2019-2032 (USD Billion)
18. U.S. Others, IVD Market, 2019-2032 (USD Billion)
List of Figures
1. U.S. IVD Market Segmentation
2. IVD Market: Research Methodology
3. Market Size Estimation Methodology: Bottom-Up Approach
4. Market Size Estimation Methodology: Top-Down Approach
5. Data Triangulation
6. Porter’s Five Forces Analysis
7. Value Chain Analysis
8. U.S. IVD Market Attractiveness Analysis By Technology
9. U.S. IVD Market Attractiveness Analysis By Application
10. U.S. IVD Market: Dynamics
11. U.S. IVD Market Share By Technology (2023 & 2032)
12. U.S. IVD Market Share by Application (2023 & 2032)
13. U.S. IVD Market Share by Company (2022)
This study forecasts revenue at the country level from 2019 to 2032. The Brainy Insights has segmented the U.S. IVD market based on below mentioned segments:
U.S. IVD Market by Technology:
U.S. IVD Market by Application:
Research has its special purpose to undertake marketing efficiently. In this competitive scenario, businesses need information across all industry verticals; the information about customer wants, market demand, competition, industry trends, distribution channels etc. This information needs to be updated regularly because businesses operate in a dynamic environment. Our organization, The Brainy Insights incorporates scientific and systematic research procedures in order to get proper market insights and industry analysis for overall business success. The analysis consists of studying the market from a miniscule level wherein we implement statistical tools which helps us in examining the data with accuracy and precision.
Our research reports feature both; quantitative and qualitative aspects for any market. Qualitative information for any market research process are fundamental because they reveal the customer needs and wants, usage and consumption for any product/service related to a specific industry. This in turn aids the marketers/investors in knowing certain perceptions of the customers. Qualitative research can enlighten about the different product concepts and designs along with unique service offering that in turn, helps define marketing problems and generate opportunities. On the other hand, quantitative research engages with the data collection process through interviews, e-mail interactions, surveys and pilot studies. Quantitative aspects for the market research are useful to validate the hypotheses generated during qualitative research method, explore empirical patterns in the data with the help of statistical tools, and finally make the market estimations.
The Brainy Insights offers comprehensive research and analysis, based on a wide assortment of factual insights gained through interviews with CXOs and global experts and secondary data from reliable sources. Our analysts and industry specialist assume vital roles in building up statistical tools and analysis models, which are used to analyse the data and arrive at accurate insights with exceedingly informative research discoveries. The data provided by our organization have proven precious to a diverse range of companies, facilitating them to address issues such as determining which products/services are the most appealing, whether or not customers use the product in the manner anticipated, the purchasing intentions of the market and many others.
Our research methodology encompasses an idyllic combination of primary and secondary initiatives. Key phases involved in this process are listed below:
The phase involves the gathering and collecting of market data and its related information with the help of different sources & research procedures.
The data procurement stage involves in data gathering and collecting through various data sources.
This stage involves in extensive research. These data sources includes:
Purchased Database: Purchased databases play a crucial role in estimating the market sizes irrespective of the domain. Our purchased database includes:
Primary Research: The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. Primary research mainly involves in telephonic interviews, E-mail interactions and face-to-face interviews with the raw material providers, manufacturers/producers, distributors, & independent consultants. The interviews that we conduct provides valuable data on market size and industry growth trends prevailing in the market. Our organization also conducts surveys with the various industry experts in order to gain overall insights of the industry/market. For instance, in healthcare industry we conduct surveys with the pharmacists, doctors, surgeons and nurses in order to gain insights and key information of a medical product/device/equipment which the customers are going to usage. Surveys are conducted in the form of questionnaire designed by our own analyst team. Surveys plays an important role in primary research because surveys helps us to identify the key target audiences of the market. Additionally, surveys helps to identify the key target audience engaged with the market. Our survey team conducts the survey by targeting the key audience, thus gaining insights from them. Based on the perspectives of the customers, this information is utilized to formulate market strategies. Moreover, market surveys helps us to understand the current competitive situation of the industry. To be precise, our survey process typically involve with the 360 analysis of the market. This analytical process begins by identifying the prospective customers for a product or service related to the market/industry to obtain data on how a product/service could fit into customers’ lives.

Secondary Research: The secondary data sources includes information published by the on-profit organizations such as World bank, WHO, company fillings, investor presentations, annual reports, national government documents, statistical databases, blogs, articles, white papers and others. From the annual report, we analyse a company’s revenue to understand the key segment and market share of that organization in a particular region. We analyse the company websites and adopt the product mapping technique which is important for deriving the segment revenue. In the product mapping method, we select and categorize the products offered by the companies catering to domain specific market, deduce the product revenue for each of the companies so as to get overall estimation of the market size. We also source data and analyses trends based on information received from supply side and demand side intermediaries in the value chain. The supply side denotes the data gathered from supplier, distributor, wholesaler and the demand side illustrates the data gathered from the end customers for respective market domain.
The supply side for a domain specific market is analysed by:
The demand side for the market is estimated through:
In-house Library: Apart from these third-party sources, we have our in-house library of qualitative and quantitative information. Our in-house database includes market data for various industry and domains. These data are updated on regular basis as per the changing market scenario. Our library includes, historic databases, internal audit reports and archives.
Sometimes there are instances where there is no metadata or raw data available for any domain specific market. For those cases, we use our expertise to forecast and estimate the market size in order to generate comprehensive data sets. Our analyst team adopt a robust research technique in order to produce the estimates:
Data Synthesis: This stage involves the analysis & mapping of all the information obtained from the previous step. It also involves in scrutinizing the data for any discrepancy observed while data gathering related to the market. The data is collected with consideration to the heterogeneity of sources. Robust scientific techniques are in place for synthesizing disparate data sets and provide the essential contextual information that can orient market strategies. The Brainy Insights has extensive experience in data synthesis where the data passes through various stages:
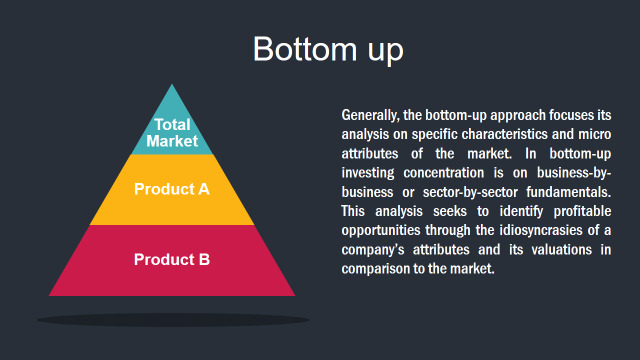
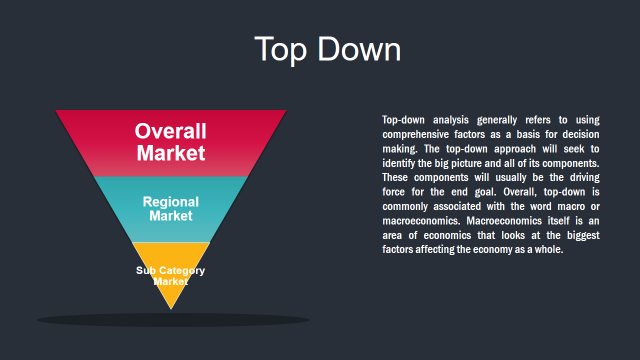
Market Deduction & Formulation: The final stage comprises of assigning data points at appropriate market spaces so as to deduce feasible conclusions. Analyst perspective & subject matter expert based holistic form of market sizing coupled with industry analysis also plays a crucial role in this stage.
This stage involves in finalization of the market size and numbers that we have collected from data integration step. With data interpolation, it is made sure that there is no gap in the market data. Successful trend analysis is done by our analysts using extrapolation techniques, which provide the best possible forecasts for the market.
Data Validation & Market Feedback: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helps us finalize data-points to be used for final calculations.
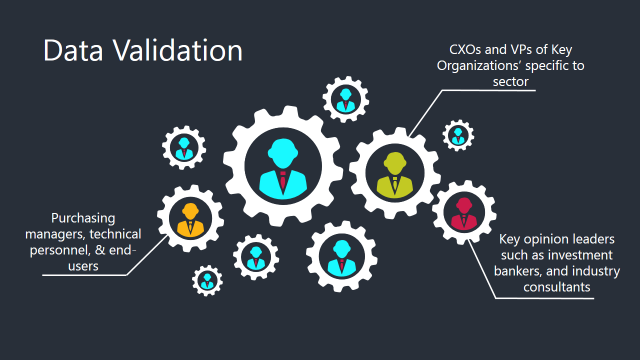
The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. The data validation interview and discussion panels are typically composed of the most experienced industry members. The participants include, however, are not limited to:
Moreover, we always validate our data and findings through primary respondents from all the major regions we are working on.
Free Customization
Fortune 500 Clients
Free Yearly Update On Purchase Of Multi/Corporate License
Companies Served Till Date