- +1-315-215-1633
- sales@thebrainyinsights.com

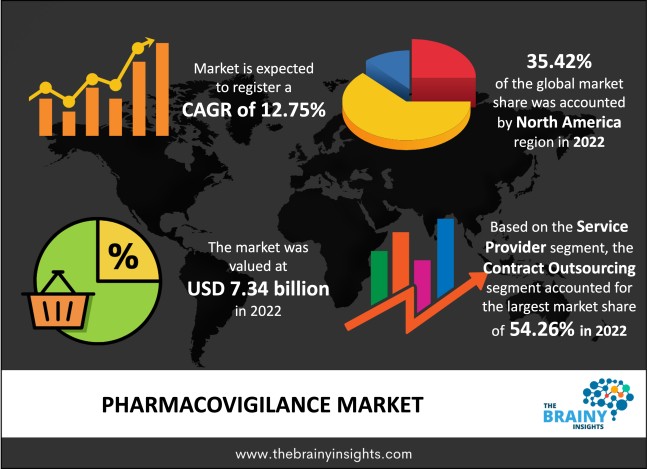
The global pharmacovigilance market was valued at USD 7.34 Billion in 2022, which is anticipated to grow at a CAGR of 12.75% from 2023 to 2032. Significant clinical trials are currently needed to create new medications due to the rising prevalence of chronic diseases like diabetes, cancer, respiratory illnesses, and cardiovascular diseases. The need for P.V. services is fueled by pharmacovigilance, which is essential for guaranteeing drug safety and keeping track of Adverse Drug Reactions (ADRs). Additionally, the market's rise is anticipated to be supported by academic activities that support pharmacovigilance (P.V.).
The prevention and treatment of diseases have changed significantly due to medicines and vaccines. Apart from their advantages, pharmaceuticals may also have side effects, some of which may be unwanted and unexpected. The research and practises surrounding identifying, evaluating, comprehending, and mitigating side effects and other issues arising from medications and vaccines are known as pharmacovigilance. Before being approved for use, all medications and vaccines undergo extensive testing for safety and efficacy during clinical trials. In contrast, the clinical trial method includes investigating these items on a few carefully chosen people for a brief period. A heterogeneous population, including those with various chronic disorders, and prolonged usage of these drugs can only lead to some adverse effects becoming prominent. Enhancing patient care and safety with a focus on medication use is the market's primary goal. Delivering trustworthy and fair information to assess drug side effects accurately also helps public health programmes. In order to provide the data points required for regulatory action, the market undertakes a number of responsibilities, including the identification, reporting, and analysis of Adverse Drug Reactions (ADRs). Additionally, the patient population's increased use of medications and rising expectations for drug safety are the main drivers of the market's rapid expansion over the coming years. Due to improvements in software and services, pharmacovigilance outsourcing is expanding and generating more income. A wide path has been opened up for the global market because of the development of an information technology platform. Over the coming years, the market is anticipated to change more rapidly. Software businesses continuously create and deploy cutting-edge platforms to ensure efficient and automated ADR reporting. ADRs are currently not being reported enough since patients are not aware that they should. Introducing mobile applications and patient-friendly software can improve ADR reporting among patients. Key drivers influencing market revenue growth include significant investments in drug development activities, a rising tendency towards outsourcing pharmacovigilance, and an increase in the prevalence of chronic diseases. The most prevalent type of heart disease, coronary heart disease, is a significant cause of death. As a result, rising demand for therapeutic drugs for the treatment of several conditions, including oncological disease and diabetes, has driven the market to rise in terms of revenue in various regions. Another crucial factor fueling market revenue increase is the rising prevalence of adverse medication responses. Due to considerable patient variability in pharmacokinetics and pharmacodynamics, most adverse drug reactions are caused by prolongation of a drug's intended pharmacologic effects.
Get an overview of this study by requesting a free sample
Growing public awareness regarding safer pharmaceuticals and outsourcing P.V. solutions- During each clinical study stage, a drug must pass the critical safety and efficacy criteria. There is a greater demand for safer pharmaceuticals because people are more aware of the adverse effects of OTC drugs. Due to the monitoring of marketed drugs for potential ADRs, this factor is projected to accelerate market expansion. Additionally, government initiatives, programmes, and pharmaceutical companies awareness campaigns significantly impact the sharing of accurate information about medications, their proper usage, adverse effects, and other topics. Hospitals and pharmaceutical companies can outsource the P.V. service in the existing market. Outsourcing offers manufacturing organisations clear advantages when it comes to time restrictions and the facilities that an outsourced service may give. Additionally, customization by client needs is possible through outsourcing, which quickens the evaluation process. These services are in greater demand than ever, which has given outsourced service providers several options. These service providers are expanding their service offerings to give customers more complex options.
Lack of consistency in adverse event reporting in low and middle-income countries- There is inconsistent reporting because patients are not aware of adverse events (A.E.). The patient typically reports suffering as an adverse event (A.E.) during an unpleasant incident. However, not all reported adverse events (A.E.s) are necessarily serious, requiring pharmaceutical corporations' committees to make poor judgements that result in the drug's withdrawal. Appropriate training, services, and software are required for these specialized services in low- and middle-income nations. Additionally, governments and pharmaceutical firms make fewer efforts to raise awareness of A.E. reporting. These factors are projected to limit market expansion in low- and middle-income nations.
The regions analyzed for the market include North America, Europe, South America, Asia Pacific, the Middle East, and Africa. North America emerged as the most significant global pharmacovigilance market, with a 35.42% market revenue share in 2022. The existence of significant market players is an essential factor in boosting market revenue growth. The expansion of pharmacovigilance efforts in numerous nations in this region is also aiding the rise of market revenue. A significant portion of the market revenue growth in this region is also attributed to significant investments in medication research. Due to the abundance of qualified healthcare professionals in the U.S., the market accounted for the biggest revenue share. In addition, significant market players in this nation are launching new products and working together to increase their market positions.
North America Region Pharmacovigilance Market Share in 2022 - 35.42%
www.thebrainyinsights.com
Check the geographical analysis of this market by requesting a free sample
The service provider segment is divided into contract outsourcing and in-house. The contract outsourcing segment dominated the market, with a market share of around 54.26% in 2022. Modern medical technology and affordability are mainly responsible for this. It is profitable for a business to select an outsourcing firm that can deliver and combine all these operations and capabilities, reducing the strain of several contracts with third parties and utilizing process synergy within the same P.V. provider. This will boost efficiency in addition to being beneficial for the business. By coordinating and carrying out safety activities in outsourcing safety in clinical trials, post-marketing observational studies, post-authorization safety studies, and other clinical or post-marketing activities, small-scale pharmaceutical companies can finish tasks without a structured, dedicated team.
The type segment is divided into intensified adverse drug reaction (ADR) reporting, cohort event monitoring, spontaneous reporting, electronic health record (EHR) mining, and targeted spontaneous reporting. The spontaneous reporting segment dominated the market, with a market share of around 23.73% in 2022. It is because it uses a method of gathering data on possible ADRs that is efficient and reasonably priced. The primary goal is to locate unique, unusual, and harmful ADRs overlooked during pre-marketing clinical trials. From the start, a medicine is introduced to the market and lasting throughout its life becomes spontaneous reporting. This strategy is also helpful in delivering data from actual clinical practice rather than clinical trials, where susceptible individuals are excluded and treatment duration is confined.
| Attribute | Description |
|---|---|
| Market Size | Revenue (USD Billion) |
| Market size value in 2022 | USD 7.34 Billion |
| Market size value in 2032 | USD 24.37 Billion |
| CAGR (2023 to 2032) | 12.75% |
| Historical data | 2019-2021 |
| Base Year | 2022 |
| Forecast | 2023-2032 |
| Region | The regions analyzed for the market are Asia Pacific, Europe, South America, North America, and Middle East & Africa. Furthermore, the regions are further analyzed at the country level. |
| Segments | Service Provider, Type |
As per The Brainy Insights, the size of the pharmacovigilance market was valued at USD 7.34 Billion in 2022 & USD 24.37 Billion by 2032.
Global pharmacovigilance market is growing at a CAGR of 12.75% during the forecast period 2023-2032.
North America region emerged as the largest market for the pharmacovigilance.
Growing public awareness regarding safer pharmaceuticals and outsourcing PV solutions is a significant driving factor for the growth of the pharmacovigilance market.
Lack of consistency in adverse event reporting in low and middle income countries is a significant restraining factor for the growth of the pharmacovigilance market.
1. Introduction
1.1. Objective of the Study
1.2. Market Definition
1.3. Research Scope
1.4. Currency
1.5. Key Target Audience
2. Research Methodology and Assumptions
3. Executive Summary
4. Premium Insights
4.1. Porter’s Five Forces Analysis
4.2. Value Chain Analysis
4.3. Top Investment Pockets
4.3.1. Market Attractiveness Analysis By Service Provider
4.3.2. Market Attractiveness Analysis By Type
4.3.3. Market Attractiveness Analysis By Region
4.4. Industry Trends
5. Market Dynamics
5.1. Market Evaluation
5.2. Drivers
5.2.1. Growing public awareness regarding safer pharmaceuticals and outsourcing PV solutions
5.3. Restraints
5.3.1. Lack of consistency in adverse event reporting in low and middle income countries
6. Global Pharmacovigilance Market Analysis and Forecast, By Service Provider
6.1. Segment Overview
6.2. Contract Outsourcing
6.3. In-House
7. Global Pharmacovigilance Market Analysis and Forecast, By Type
7.1. Segment Overview
7.2. Intensified Adverse Drug Reaction (ADR) Reporting
7.3. Cohort Event Monitoring
7.4. Spontaneous Reporting
7.5. Electronic Health Record (EHR) Mining
7.6. Targeted Spontaneous Reporting
8. Global Pharmacovigilance Market Analysis and Forecast, By Regional Analysis
8.1. Segment Overview
8.2. North America
8.2.1. U.S.
8.2.2. Canada
8.2.3. Mexico
8.3. Europe
8.3.1. Germany
8.3.2. France
8.3.3. U.K.
8.3.4. Italy
8.3.5. Spain
8.4. Asia-Pacific
8.4.1. Japan
8.4.2. China
8.4.3. India
8.5. South America
8.5.1. Brazil
8.6. Middle East and Africa
8.6.1. UAE
8.6.2. South Africa
9. Global Pharmacovigilance Market-Competitive Landscape
9.1. Overview
9.2. Market Share of Key Players in the Pharmacovigilance Market
9.2.1. Global Company Market Share
9.2.2. North America Company Market Share
9.2.3. Europe Company Market Share
9.2.4. APAC Company Market Share
9.3. Competitive Situations and Trends
9.3.1. Product Launches and Developments
9.3.2. Partnerships, Collaborations, and Agreements
9.3.3. Mergers & Acquisitions
9.3.4. Expansions
10. Company Profiles
10.1. IQVIA
10.1.1. Business Overview
10.1.2. Company Snapshot
10.1.3. Company Market Share Analysis
10.1.4. Company Product Portfolio
10.1.5. Recent Developments
10.1.6. SWOT Analysis
10.2. Clinquest Group B.V. (Linical Americas)
10.2.1. Business Overview
10.2.2. Company Snapshot
10.2.3. Company Market Share Analysis
10.2.4. Company Product Portfolio
10.2.5. Recent Developments
10.2.6. SWOT Analysis
10.3. Laboratory Corporation of America Holdings
10.3.1. Business Overview
10.3.2. Company Snapshot
10.3.3. Company Market Share Analysis
10.3.4. Company Product Portfolio
10.3.5. Recent Developments
10.3.6. SWOT Analysis
10.4. Capgemini
10.4.1. Business Overview
10.4.2. Company Snapshot
10.4.3. Company Market Share Analysis
10.4.4. Company Product Portfolio
10.4.5. Recent Developments
10.4.6. SWOT Analysis
10.5. ICON plc.
10.5.1. Business Overview
10.5.2. Company Snapshot
10.5.3. Company Market Share Analysis
10.5.4. Company Product Portfolio
10.5.5. Recent Developments
10.5.6. SWOT Analysis
10.6. Parexel International Corp.
10.6.1. Business Overview
10.6.2. Company Snapshot
10.6.3. Company Market Share Analysis
10.6.4. Company Product Portfolio
10.6.5. Recent Developments
10.6.6. SWOT Analysis
10.7. United BioSource LLC
10.7.1. Business Overview
10.7.2. Company Snapshot
10.7.3. Company Market Share Analysis
10.7.4. Company Product Portfolio
10.7.5. Recent Developments
10.7.6. SWOT Analysis
10.8. ClinChoice (formerly FMD K&L)
10.8.1. Business Overview
10.8.2. Company Snapshot
10.8.3. Company Market Share Analysis
10.8.4. Company Product Portfolio
10.8.5. Recent Developments
10.8.6. SWOT Analysis
10.9. Accenture
10.9.1. Business Overview
10.9.2. Company Snapshot
10.9.3. Company Market Share Analysis
10.9.4. Company Product Portfolio
10.9.5. Recent Developments
10.9.6. SWOT Analysis
10.10. IBM Corp.
10.10.1. Business Overview
10.10.2. Company Snapshot
10.10.3. Company Market Share Analysis
10.10.4. Company Product Portfolio
10.10.5. Recent Developments
10.10.6. SWOT Analysis
10.11. ITClinical
10.11.1. Business Overview
10.11.2. Company Snapshot
10.11.3. Company Market Share Analysis
10.11.4. Company Product Portfolio
10.11.5. Recent Developments
10.11.6. SWOT Analysis
10.12. Wipro Ltd.
10.12.1. Business Overview
10.12.2. Company Snapshot
10.12.3. Company Market Share Analysis
10.12.4. Company Product Portfolio
10.12.5. Recent Developments
10.12.6. SWOT Analysis
10.13. Cognizant
10.13.1. Business Overview
10.13.2. Company Snapshot
10.13.3. Company Market Share Analysis
10.13.4. Company Product Portfolio
10.13.5. Recent Developments
10.13.6. SWOT Analysis
10.14. TAKE Solutions Ltd.
10.14.1. Business Overview
10.14.2. Company Snapshot
10.14.3. Company Market Share Analysis
10.14.4. Company Product Portfolio
10.14.5. Recent Developments
10.14.6. SWOT Analysis
10.15. ArisGlobal
10.15.1. Business Overview
10.15.2. Company Snapshot
10.15.3. Company Market Share Analysis
10.15.4. Company Product Portfolio
10.15.5. Recent Developments
10.15.6. SWOT Analysis
10.16. BioClinica Inc.
10.16.1. Business Overview
10.16.2. Company Snapshot
10.16.3. Company Market Share Analysis
10.16.4. Company Product Portfolio
10.16.5. Recent Developments
10.16.6. SWOT Analysis
List of Table
1. Global Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
2. Global Contract Outsourcing, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
3. Global In-House, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
4. Global Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
5. Global Intensified Adverse Drug Reaction (ADR) Reporting, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
6. Global Cohort Event Monitoring, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
7. Global Spontaneous Reporting, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
8. Global Electronic Health Record (EHR) Mining, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
9. Global Targeted Spontaneous Reporting, Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
10. Global Pharmacovigilance Market, By Region, 2019-2032 (USD Billion)
11. North America Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
12. North America Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
13. U.S. Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
14. U.S. Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
15. Canada Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
16. Canada Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
17. Mexico Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
18. Mexico Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
19. Europe Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
20. Europe Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
21. Germany Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
22. Germany Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
23. France Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
24. France Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
25. U.K. Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
26. U.K. Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
27. Italy Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
28. Italy Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
29. Spain Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
30. Spain Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
31. Asia Pacific Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
32. Asia Pacific Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
33. Japan Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
34. Japan Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
35. China Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
36. China Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
37. India Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
38. India Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
39. South America Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
40. South America Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
41. Brazil Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
42. Brazil Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
43. Middle East and Africa Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
44. Middle East and Africa Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
45. UAE Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
46. UAE Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
47. South Africa Pharmacovigilance Market, By Service Provider, 2019-2032 (USD Billion)
48. South Africa Pharmacovigilance Market, By Type, 2019-2032 (USD Billion)
List of Figures
1. Global Pharmacovigilance Market Segmentation
2. Pharmacovigilance Market: Research Methodology
3. Market Size Estimation Methodology: Bottom-Up Approach
4. Market Size Estimation Methodology: Top-Down Approach
5. Data Triangulation
6. Porter’s Five Forces Analysis
7. Value Chain Analysis
8. Global Pharmacovigilance Market Attractiveness Analysis By Service Provider
9. Global Pharmacovigilance Market Attractiveness Analysis By Type
10. Global Pharmacovigilance Market Attractiveness Analysis By Region
11. Global Pharmacovigilance Market: Dynamics
12. Global Pharmacovigilance Market Share by Service Provider (2023 & 2032)
13. Global Pharmacovigilance Market Share by Type (2023 & 2032)
14. Global Pharmacovigilance Market Share by Regions (2023 & 2032)
15. Global Pharmacovigilance Market Share by Company (2022)
This study forecasts revenue at global, regional, and country levels from 2019 to 2032. The Brainy Insights has segmented the global pharmacovigilance market based on below mentioned segments:
Global Pharmacovigilance by Service Provider:
Global Pharmacovigilance by Type:
Global Pharmacovigilance by Region:
Research has its special purpose to undertake marketing efficiently. In this competitive scenario, businesses need information across all industry verticals; the information about customer wants, market demand, competition, industry trends, distribution channels etc. This information needs to be updated regularly because businesses operate in a dynamic environment. Our organization, The Brainy Insights incorporates scientific and systematic research procedures in order to get proper market insights and industry analysis for overall business success. The analysis consists of studying the market from a miniscule level wherein we implement statistical tools which helps us in examining the data with accuracy and precision.
Our research reports feature both; quantitative and qualitative aspects for any market. Qualitative information for any market research process are fundamental because they reveal the customer needs and wants, usage and consumption for any product/service related to a specific industry. This in turn aids the marketers/investors in knowing certain perceptions of the customers. Qualitative research can enlighten about the different product concepts and designs along with unique service offering that in turn, helps define marketing problems and generate opportunities. On the other hand, quantitative research engages with the data collection process through interviews, e-mail interactions, surveys and pilot studies. Quantitative aspects for the market research are useful to validate the hypotheses generated during qualitative research method, explore empirical patterns in the data with the help of statistical tools, and finally make the market estimations.
The Brainy Insights offers comprehensive research and analysis, based on a wide assortment of factual insights gained through interviews with CXOs and global experts and secondary data from reliable sources. Our analysts and industry specialist assume vital roles in building up statistical tools and analysis models, which are used to analyse the data and arrive at accurate insights with exceedingly informative research discoveries. The data provided by our organization have proven precious to a diverse range of companies, facilitating them to address issues such as determining which products/services are the most appealing, whether or not customers use the product in the manner anticipated, the purchasing intentions of the market and many others.
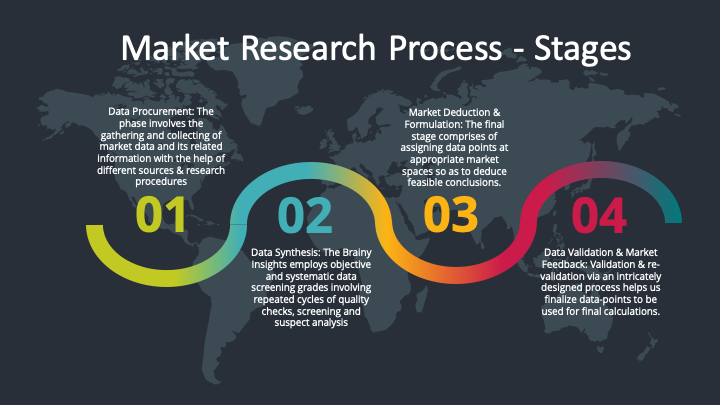
Our research methodology encompasses an idyllic combination of primary and secondary initiatives. Key phases involved in this process are listed below:
The phase involves the gathering and collecting of market data and its related information with the help of different sources & research procedures.
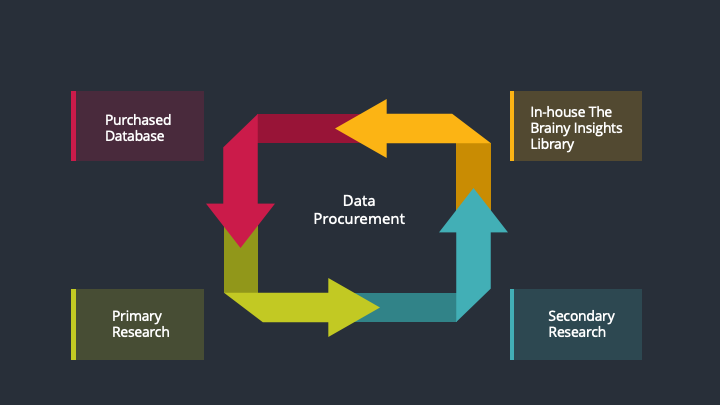
The data procurement stage involves in data gathering and collecting through various data sources.
This stage involves in extensive research. These data sources includes:
Purchased Database: Purchased databases play a crucial role in estimating the market sizes irrespective of the domain. Our purchased database includes:
Primary Research: The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. Primary research mainly involves in telephonic interviews, E-mail interactions and face-to-face interviews with the raw material providers, manufacturers/producers, distributors, & independent consultants. The interviews that we conduct provides valuable data on market size and industry growth trends prevailing in the market. Our organization also conducts surveys with the various industry experts in order to gain overall insights of the industry/market. For instance, in healthcare industry we conduct surveys with the pharmacists, doctors, surgeons and nurses in order to gain insights and key information of a medical product/device/equipment which the customers are going to usage. Surveys are conducted in the form of questionnaire designed by our own analyst team. Surveys plays an important role in primary research because surveys helps us to identify the key target audiences of the market. Additionally, surveys helps to identify the key target audience engaged with the market. Our survey team conducts the survey by targeting the key audience, thus gaining insights from them. Based on the perspectives of the customers, this information is utilized to formulate market strategies. Moreover, market surveys helps us to understand the current competitive situation of the industry. To be precise, our survey process typically involve with the 360 analysis of the market. This analytical process begins by identifying the prospective customers for a product or service related to the market/industry to obtain data on how a product/service could fit into customers’ lives.

Secondary Research: The secondary data sources includes information published by the on-profit organizations such as World bank, WHO, company fillings, investor presentations, annual reports, national government documents, statistical databases, blogs, articles, white papers and others. From the annual report, we analyse a company’s revenue to understand the key segment and market share of that organization in a particular region. We analyse the company websites and adopt the product mapping technique which is important for deriving the segment revenue. In the product mapping method, we select and categorize the products offered by the companies catering to domain specific market, deduce the product revenue for each of the companies so as to get overall estimation of the market size. We also source data and analyses trends based on information received from supply side and demand side intermediaries in the value chain. The supply side denotes the data gathered from supplier, distributor, wholesaler and the demand side illustrates the data gathered from the end customers for respective market domain.
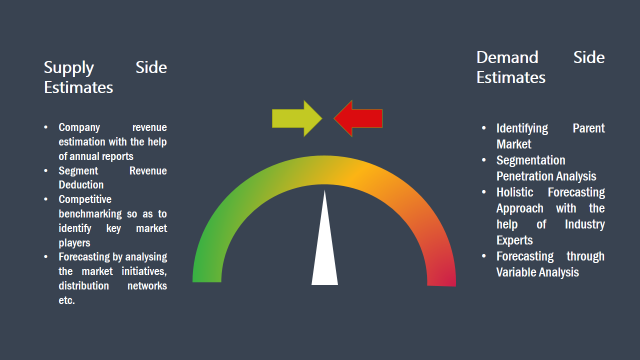
The supply side for a domain specific market is analysed by:
The demand side for the market is estimated through:
In-house Library: Apart from these third-party sources, we have our in-house library of qualitative and quantitative information. Our in-house database includes market data for various industry and domains. These data are updated on regular basis as per the changing market scenario. Our library includes, historic databases, internal audit reports and archives.
Sometimes there are instances where there is no metadata or raw data available for any domain specific market. For those cases, we use our expertise to forecast and estimate the market size in order to generate comprehensive data sets. Our analyst team adopt a robust research technique in order to produce the estimates:
Data Synthesis: This stage involves the analysis & mapping of all the information obtained from the previous step. It also involves in scrutinizing the data for any discrepancy observed while data gathering related to the market. The data is collected with consideration to the heterogeneity of sources. Robust scientific techniques are in place for synthesizing disparate data sets and provide the essential contextual information that can orient market strategies. The Brainy Insights has extensive experience in data synthesis where the data passes through various stages:
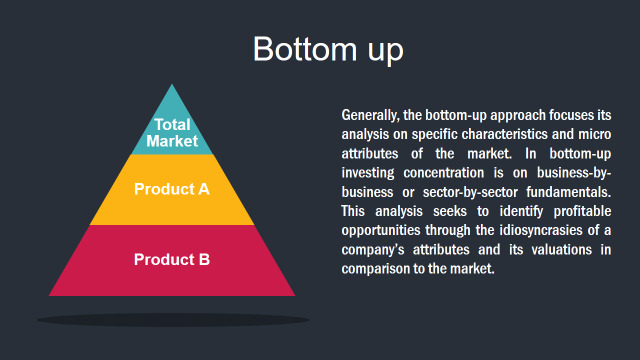
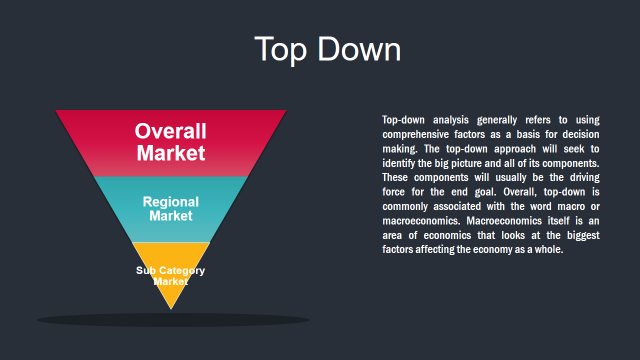
Market Deduction & Formulation: The final stage comprises of assigning data points at appropriate market spaces so as to deduce feasible conclusions. Analyst perspective & subject matter expert based holistic form of market sizing coupled with industry analysis also plays a crucial role in this stage.
This stage involves in finalization of the market size and numbers that we have collected from data integration step. With data interpolation, it is made sure that there is no gap in the market data. Successful trend analysis is done by our analysts using extrapolation techniques, which provide the best possible forecasts for the market.
Data Validation & Market Feedback: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helps us finalize data-points to be used for final calculations.
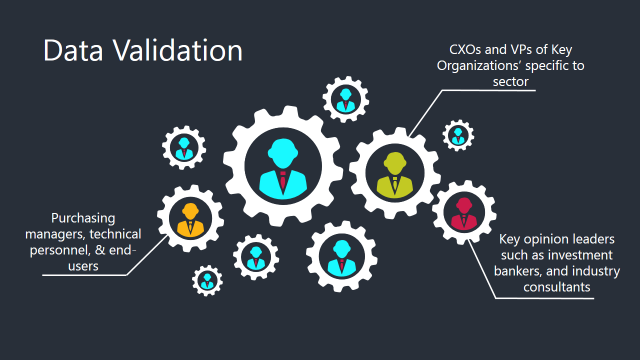
The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. The data validation interview and discussion panels are typically composed of the most experienced industry members. The participants include, however, are not limited to:
Moreover, we always validate our data and findings through primary respondents from all the major regions we are working on.
Free Customization
Fortune 500 Clients
Free Yearly Update On Purchase Of Multi/Corporate License
Companies Served Till Date